Tailoring photoluminescence stability in double perovskite red phosphors A2BAlF6:Mn4+ (A = Rb, Cs; B = K, Rb) via neighboring-cation modulation†
Abstract
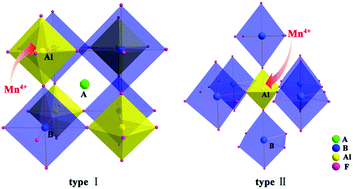
Three isotypic double perovskites A2BAlF6:Mn4+ (A = Rb, Cs; B = K, Rb) were synthesized via a facile co-precipitation method at room temperature. XRD Rietveld refinements and Raman spectra indicate that the neighboring smaller A-site or larger B-site cation in A2BAlF6:Mn4+ lowers structural stability, but suppresses the [AlF6] octahedron to increase the crystal field strength and bond strength of the Al–F bond. Cs2KAlF6:Mn4+ presents a sharp line red light and excellent moisture resistance, but experiences severe thermal quenching. In comparison, altering the A- or B-site cation to Rb, Rb2KAlF6:Mn4+ and Cs2RbAlF6:Mn4+ shows blue shifts in emission and declining water resistance, but enhancements in thermal stability. A combination of static and dynamic emission degradations demonstrates that the water resistance of Mn4+ emission mainly relies on the structural stability of the host matrix; meanwhile, the thermal stability highly depends on the rigidity of its local accommodation. The variations of the temperature-dependent white light-emitting diode (WLED) performances further evidence their sufficient thermal stability for LED applications. These findings suggest that neighboring-cation modulation might be a feasible guideline for exploiting stable Mn4+-activated fluoride red phosphors in warm WLED applications.


 Please wait while we load your content...
Please wait while we load your content...