Near-UV-to-red light conversion through energy transfer in Ca2Sr(PO4)2:Ce3+,Mn2+ for plant growth
Abstract
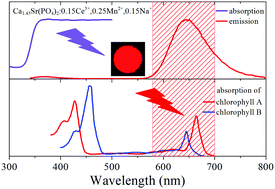
A series of Ca2Sr(PO4)2:Ce3+,Mn2+,Na+ phosphors were synthesized by a high-temperature solid-state reaction. Under ∼320 nm excitation, the Ce3+ and Mn2+ co-doped phosphors exhibit two emission bands peaking at 370 and 645 nm, which originate from 5d–4f and 3d–3d transitions of Ce3+ and Mn2+, respectively. Luminescence properties, diffuse reflectance spectra, and decay curves indicate that energy transfer (ET) occurs from Ce3+ to Mn2+, and the ET efficiency reaches its maximum (91%) at an Mn2+ content of 0.35. The diffuse reflectance spectrum shows that the co-doped phosphors have strong absorption around 320 nm, which is good for the anti-aging ability of an agricultural film. The absolute quantum efficiency of the Ca1.6Sr(PO4)2:0.15Ce3+,0.10Mn2+,0.15Na+ phosphor is ∼94%. And the co-doped phosphors exhibit good stability in water. Therefore, Ca2Sr(PO4)2:Ce3+,Mn2+,Na+ phosphors have potential application as a light conversion material in agricultural films.


 Please wait while we load your content...
Please wait while we load your content...