Anion-induced ferroelectric polarization in a luminescent metal–organic cage compound†
Abstract
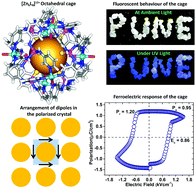
Metal–organic crystalline solids with ferroelectric properties have attracted significant attention recently as materials for high-tech applications. Here, we describe two crystalline assemblies that contain cationic metal–organic cages {[Zn6(H2O)12][TPTA]8}(NO3)12·26H2O (1) and {[Zn6(H2O)12][TPTA]8}(ClO4)12·18.75H2O (2) featuring the tripodal ligand [PS(NH3Py)3] (TPTA). Ferroelectric measurement on a single crystal of 1 gave a remnant (Pr) polarization of 1.2 μC cm−2 at room temperature. The ferroelectric response originates from the toggling of nitrate anions and solvate molecules found in pockets between the cages. The temperature-dependent permittivity of 1 shows an anomalous dielectric peak at 20 °C. This is attributed to the desolvation-assisted dielectric relaxation behaviour and signifies the role of solvate molecules in the ferroelectric behaviour of 1. In addition, this material is highly luminescent exhibiting a bright blue emission under UV light. This multifunctional behaviour is unique among metal–organic cage frameworks.


 Please wait while we load your content...
Please wait while we load your content...