Highly-efficient solid-state emissions of anthracene–o-carborane dyads with various substituents and their thermochromic luminescence properties†
Abstract
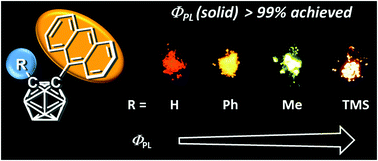
This manuscript describes the synthesis and optical properties of a series of anthracenyl-o-carborane dyads exhibiting highly-efficient luminescence with various types of substituent groups at the adjacent carbon atom of o-carborane. The restricted rotational motion of the anthracene moiety and the ideal orientation for intramolecular charge transfer from the anthracene moiety to the carborane cluster resulted in yellow and orange emissions (λem = 563 nm and 604 nm) with approximately 100% absolute fluorescence quantum efficiencies in the crystalline state of the methyl- and trimethylsilyl (TMS)-substituted dyads, respectively. In additional, clear thermochromic luminescence properties were also observed. Computer calculations were carried out to investigate the influence of the substituent effect on emission efficiency.


 Please wait while we load your content...
Please wait while we load your content...