Dimerization effect of fluorene-based semiconductors on conformational planarization for microcrystal lasing†
Abstract
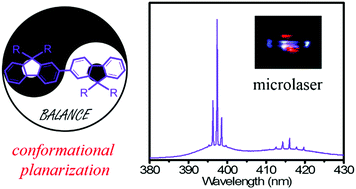
Dimerization with a flexible rotation of bonds between repeat monomers is an effective platform to clarify the structure–property relationship for potential optoelectronic applications of organic semiconductors. Herein, four diarylfluorene dimers were designed and synthesized to investigate the conformational behaviors and their effect on the photophysical properties. Surprisingly, all the dimers adopted fully planar and trans conformation with a torsion angle of 180°, indicating that the steric bulks in bifluorenes could not disrupt the formation ability of the coplanar conformation (also called β phase in polyfluorenes). These results also suggest that the presence of alkyl side chains is not a necessary condition to induce conformation planarization in fluorene-based semiconductors. In addition, large spectral redshift (more than 30 nm) in absorption but similar photoexcited properties between the pristine amorphous and annealed films of BSBF and DSFXSO clearly indicate that excited-state conformational planarization determines the luminescent properties in amorphous films. Finally, the regularly shaped microcrystals of BDPhF and BSBF show ultraviolet lasing with a threshold of 46.5 and 134.8 W cm−2, respectively, further confirming that these dimers can serve as efficient gain media for organic microlasers.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...