A Zn2+-coordinated boronate dipyrrin as a chemodosimeter toward hydrogen peroxide†
Abstract
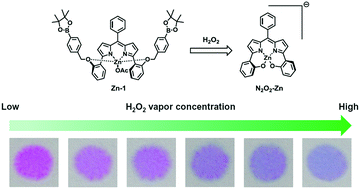
Driven by our motivation to develop a chemodosimeter for the visual detection of hydrogen peroxide (H2O2), we synthesized for the first time a dipyrrin (1) with 4-pinacolborylbenzyloxy groups. An EtOH solution of Zn2+-coordinated 1 was reddish-violet in color, with a λmax value of 538 nm. However, the addition of H2O2 into the solution induced a remarkable color change from reddish-violet to blue. The time-dependent change in the absorption band showed two step kinetic processes in their kinetic profiles, where a new peak with λmax = 581 nm appeared within 13 min (k1 = 0.370 min−1), followed by a slower change in the spectra (k2 = 0.00371 min−1) concomitant with a further bathochromic shift of 18 nm. NMR and mass spectroscopic analyses indicated that the kinetically slower process may be responsible for a change in the coordination mode of the Zn2+ ion through a H2O2-triggered self-immolative reaction in the 4-pinacolborylbenzyloxy groups. Basic conditions achieved through the addition of tetrabutylammonium hydroxide (TBAOH) led to the acceleration of not only the H2O2-triggered colorimetric change but also the turn-on fluorescence, thus allowing dual-mode sensing of H2O2. For practical applications, the related drop-cast paper strips were fabricated to visually detect H2O2 vapor.


 Please wait while we load your content...
Please wait while we load your content...