Zn(ii)-based metal–organic framework: an exceptionally thermally stable, guest-free low dielectric material†
Abstract
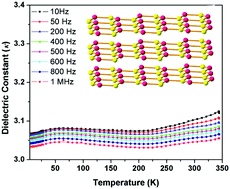
The synthesis of an exceptionally thermally stable, chemically stable, guest-free low-κ dielectric metal–organic coordination framework [Zn2(Hbbim)2(bbim)]n (1, H2bbim = bisbenzimidazole) was achieved under hydrothermal conditions. Structural analysis showed that compound 1 crystallizes in a triclinic space group P1 and possesses a guest-free structure. Compound 1 was found to be a low-κ dielectric material (3.05 at 1 MHz) that was extremely robust towards various solvents. The compound was exceptionally thermally stable and retained its structure at temperatures of up to 450 °C. Temperature-dependent dielectric studies revealed that 1 has a low-dielectric constant that remains stable upon increasing the temperature. This low-dielectric constant was further supported by density functional theory calculations, which showed that the dielectric property can be mainly attributed to electronic polarizability.


 Please wait while we load your content...
Please wait while we load your content...