Electronic structure of silicene: effects of the organic molecular adsorption and substrate
Abstract
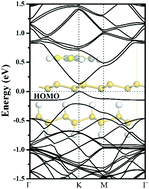
The potential of silicene-based integrated electronics originates from its extremely high carrier mobility, whereas the lack of a band gap impedes its application. Thus, opening a sizeable band gap without degrading its carrier mobility is a significant challenge for application in logic circuits. In this study, a sizable band gap is created in silicene by the dual effect of organic molecule adsorption and a substrate. As an electron donor molecule, tetrathiafulvalene (TTF) is found to non-covalently functionalize the silicene sheet. As a result, silicene with adsorbed TTF exhibits an open band gap. When silicane (hydrogenated silicene) substrate is applied, the band gap further widens. Moreover, the high carrier mobility is largely retained. These results provide effective and reversible routes for engineering the band gap of silicene.


 Please wait while we load your content...
Please wait while we load your content...