The atomic structures and electronic properties of potassium-doped phenanthrene from a first-principles study
Abstract
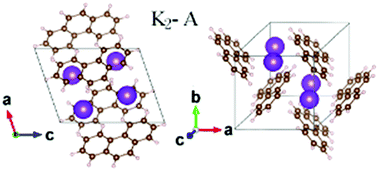
The atomic structures and electronic properties of potassium-doped phenanthrene (PHA) with various doping levels have been investigated using first principles calculations based on van der Waals density functional theory (DFT). Our results show that the possible stoichiometric content of potassium in KxPHA compounds is x = 1 or x = 2, corresponding to the structural phases K1-A, K1-B or K2-A. The formation energy of the K2-A phase is −0.32 eV per K atom, which is comparable to the energy of KC8. These K3PHA phases, mentioned in previous reports, have a positive formation energy larger than +0.24 eV per K atom, which should not exist in samples. K2PHA in the K2-A phase is a metal with three bands crossing the Fermi level, and the Fermi surface sheet along Γ–Z has a cylinder shape associated with the two-dimensional nature of the electronic states. Our calculations suggest that the K2-A phase is the most stable and reasonable structure in K-doped PHA compounds.


 Please wait while we load your content...
Please wait while we load your content...