Mechanochromic asymmetric sulfone derivatives for use in efficient blue organic light-emitting diodes†
Abstract
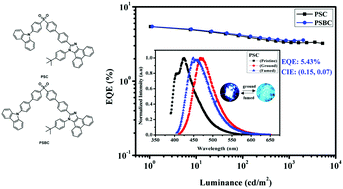
Typical π–π stacking is suppressed by the asymmetric molecular design for high fluorescence quantum yield (Φf) blue light emission, overcoming the aggregation caused quenching (ACQ) limitation. In this research, two novel blue fluorescent materials with asymmetric structure: 2-(4′-((4-(9H-carbazol-9-yl)phenyl)sulfonyl)-[1,1′-biphenyl]-4-yl)-1-(4-(tert-butyl)phenyl)-1H-phenanthro[9,10-d]imidazole (PSC) and 2-(4′-((4′-(9H-carbazol-9-yl)-[1,1′-biphenyl]-4-yl)sulfonyl)-[1,1′-biphenyl]-4-yl)-1-(4-(tert-butyl)phenyl)-1H-phenanthro[9,10-d]imidazole (PSBC), consisting of a sulfone group as the electron acceptor and two different electron donors, carbazole and phenanthroimidazole, were designed and synthesized. The two compounds have high Φf (95.3% for PSC and 81.1% for PSBC) in film because of the restricting π–π stacking, and show apparent mechanochromic properties, i.e., an emission change from deep blue to blue-green resulting from external mechanical stimuli. The emissions display 50 nm/23 nm red shifts after grinding. Organic light emitting diodes (OLEDs) using the two compounds as emitters exhibited good efficiencies: the doped PSC-based device emitted blue light at 444 nm with CIE co-ordinates of (0.151, 0.072). The PSBC-based device also emitted blue light at 444 nm with CIE co-ordinates of (0.151, 0.068). A maximal external quantum efficiency (EQE) of 5.43% was also achieved.

 Please wait while we load your content...
Please wait while we load your content...