Tuning charge transport from unipolar (n-type) to ambipolar in bis(naphthalene diimide) derivatives by introducing π-conjugated heterocyclic bridging moieties†
Abstract
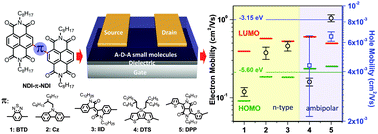
A series of acceptor–donor–acceptor (A–D–A) conjugated molecules based on naphthalene diimide dimers bridged with different π-conjugated heterocyclic units (NDI–π–NDI) have been designed and synthesized. By an ingenious design strategy, the LUMO (the lowest unoccupied molecular orbital) of the NDI-based small molecules is well controlled to a relatively constant value of −3.8 to −3.9 eV, whereas their HOMO (the highest occupied molecular orbital) could be tuned over a wide range, from −6.5 eV (compound 1) to −5.5 eV (compound 5), leading to varied band gaps from 2.6 eV to 1.5 eV. Organic field-effect transistor (OFET) characterization of these NDI–π–NDI molecules shows that compounds 1, 2, and 3 have good n-type semiconducting properties in a N2 atmosphere with the maximum electron mobilities up to 0.15 cm2 V−1 s−1, 0.46 cm2 V−1 s−1 and 0.57 cm2 V−1 s−1, respectively. Compounds 4 and 5, due to the high-lying HOMO levels and reduced energy band gaps, have ambipolar semiconducting properties and OFETs based on 5 show the highest electron and hole mobilities up to 1.23 cm2 V−1 s−1 and 0.0074 cm2 V−1 s−1, respectively. Moreover, the performances are enhanced under thermal treatment because of the increased crystallinity as evidenced by X-ray diffraction (XRD) and atomic force microscopy (AFM). The easily tunable electronic energy levels make the NDI-based semiconductors promising n-channel and ambipolar components in organic devices.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry C Hot Papers

 Please wait while we load your content...
Please wait while we load your content...