D–A–D type chromophores with aggregation-induced emission and two-photon absorption: synthesis, optical characteristics and cell imaging†
Abstract
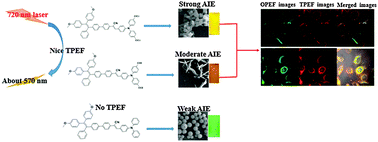
Three novel D–A–D type chromophores, consisting of substituted triphenylamine and aggregation-induced emission (AIE)-active tetraphenylethylene moieties, 1 (with two ethoxy groups), 2 (with two hydroxyl groups), 3 (non-substituted), were synthesized and characterized. Their one-photon excited fluorescence (OPEF) quantum yields (ΦF) and lifetimes (τ) in organic solutions increased with the increase of the electron-donating ability of the substituents on the triphenylamine unit, i.e., 1 > 2 > 3. By means of dynamic light scattering and scanning electron microscopy, it was found that the higher hydrophobicity of 1 resulted in larger particle sizes and a strong AIE effect. 1 and 2 showed good two-photon excited fluorescence (TPEF) and two-photon absorption cross-sections (σ) (4230 and 4004, respectively) and proved to have good biocompatibility. Both compounds 1 and 2 were successfully applied in cell imaging based on their OPEF and TPEF properties. This work clearly implies that these chromophores can be finely tuned for different optical properties and applications through varying the electron-donating ability, hydrophobicity, and cytotoxicity of the substituents.

 Please wait while we load your content...
Please wait while we load your content...