Facile preparation of copper nitride powders and nanostructured films†
Abstract
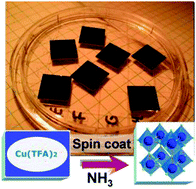
The simple fluorinated precursor, copper(II) trifluoroacetate, Cu(CF3COO)2 can be effectively utilised in the synthesis of copper(I) nitride, Cu3N, powders and films by combinations of wet processing and gas–solid (ammonolysis) techniques. The resulting materials were characterized by powder X-ray diffraction (PXD), scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDX), atomic force microscopy (AFM), diffuse reflectance UV-visible spectroscopy (DRUV-Vis), Raman spectroscopy, infrared spectroscopy (IR), thermogravimetric-differential thermal analysis-mass spectrometry (TG-DTA-MS) and nitrogen adsorption (BET) analysis. Moreover, variable temperature IR (VT-IR) studies of the solid phase were performed in situ during ammonolysis. Single-phase Cu3N powders composed of sub-micron scale platelets can be produced over relatively short reaction times. Materials prepared in this way are stoichiometric narrow band gap semiconductors. The same trifluoroacetate precursor was used to prepare nanostructured nitride films by spin coating. The surface microstructure was investigated and evaluated relative to films deposited by dip coating and nebulisation using the soluble carboxylate precursor.

 Please wait while we load your content...
Please wait while we load your content...