Thienylphenothiazine integrated pyrenes: an account on the influence of substitution patterns on their optical and electroluminescence properties†
Abstract
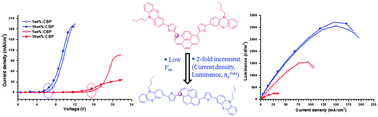
A new set of thienylphenothiazine-integrated pyrenes have been prepared by Stille coupling reactions in good yields and characterized by optical, electrochemical, thermal and electroluminescence investigations. The optical properties of these dyes are highly dependent on the chromophore density on the pyrene nucleus. Thus, the tetra-substituted derivative displayed the most red-shifted absorption and emission profiles among the series, indicative of an extended π-conjugation length. And the molar extinction coefficients increased progressively from mono- to tetra-substitution, attributable to the increment in the chromophore density. Interestingly, the physicochemical properties of the isomers such as 1,6- and 1,8-disubstituted derivatives remained unaltered by positional tuning. The emission profiles of the dyes are tuned from the cyan to the orange region in accordance with the chromophore substitution. All the dyes displayed positive solvatochromism in the emission spectra, whereas their absorption profiles are insensitive to solvent polarity, indicative of a more polarized excited state of the dyes. These dyes exhibited exceptional thermal stability with remarkable thermal decomposition temperatures, which fell in the range of 585–669 °C. The oxidation propensity of these dyes increases with increasing phenothiazine loading, while the LUMO is well stabilized by tetra-substitution. Solution processable multilayer OLEDs were fabricated by using the selected dyes featuring mono-, di- and tetra-substitution either as host emitters or dopants in the CBP host matrix and found to exhibit decent device performances. Intriguingly, the 1,6-substituted isomer exhibited superior performance over the 1,8-substituted isomer.


 Please wait while we load your content...
Please wait while we load your content...