Lanthanide metal–organic frameworks assembled from a fluorene-based ligand: selective sensing of Pb2+ and Fe3+ ions†
Abstract
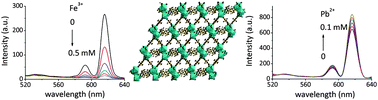
Fast and selective detection of heavy metal ions in the aqueous phase plays a key role in meeting human health and environmental concerns. Herein we report a series of fluorene-based lanthanide metal–organic frameworks ([Ln2(FDC)3DMA(H2O)3]·DMA·4.5H2O, Ln = Sm (1), Eu (2), Gd (3) and Tb (4), H2FDC = 9,9-dimethyl-2,7-fluorenedicarboxylic acid, DMA = dimethylacetamide). Single crystal X-ray diffraction reveals that 1–4 are isostructural and display a 3D neutral framework. 2 exhibits intense characteristic red emission of Eu3+ ions in the solid state and high selectivity for Pb2+ and Fe3+ ions through fluorescence enhancement and the quenching effect in aqueous solutions, respectively. Interestingly, the fluorescence intensity of 2 shows a good linear relationship with Pb2+ concentration in the range of 0.02–0.1 mM. Furthermore, the dynamic and static quenching constants are calculated to be 320 M−1 and 10 680 M−1 by the fluorescence lifetime and titration experiments in low concentration of Fe3+. In addition, 4 exhibits different fluorescence response behaviors in the presence of Sm3+ or Eu3+ in aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...