7,7′-Diazaisoindigo: a novel building block for organic electronics†
Abstract
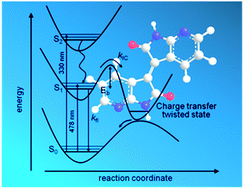
In this paper, a new family of 7,7′-diazaisoindigo molecules was synthesized and the electronic properties of these materials were studied by theoretical calculations and photophysical studies. Density functional theory (DFT) calculations at the B3LYP/6-311+G** level of theory demonstrate that the diaza-substitution clearly imposes a higher planarity in these molecules compared to the isoindigo counterparts. This effect is ascribed to an electrostatic attraction between the carbonyl group and the H atom at position 4. The isodensity surfaces and energies of the frontier molecular orbitals (HOMO and LUMO) calculated at the B3LYP/6-311++G(2d,p) level of theory show a stabilization (∼0.35 eV) of both orbitals in the 7,7′-diazaisoindigo derivatives compared to isoindigo and also the intramolecular charge-transfer character for the HOMO → LUMO transition, as it occurs in isoindigo. Photophysical studies were carried out using steady-state and time-resolved picosecond fluorescence techniques. The emission spectra show a red-shift of the peak upon increasing the polarity of the solvents which confirms the charge-transfer character of this transition. Moreover, the intensity of the emission peak decreases with the solvent polarity and increases in viscous solvents, which is tentatively attributed to a non-radiative deactivation pathway connected with a torsion of the central ethylene bond. The time-resolved emission technique shows shorter fluorescence lifetimes with increasing polarity of the solvents, in line with the stronger quenching of the fluorescence signal. The appearance of fluorescence peaks and relatively long fluorescence lifetimes in the 7,7′-diazaisoindigo derivatives compared to the non-fluorescent isoindigo counterpart are ascribed to the fine adjustment of the orbital energies through the aza insertion in the isoindigo core structure. This is a consequence of the slower non-radiative process in 7,7′-diazaisoindigo which may help to promote the use of this building block instead of isoindigo in organic electronics.

 Please wait while we load your content...
Please wait while we load your content...