Modulation of the fluorescence properties of diketopyrrolopyrroles via various electron-rich substituents†
Abstract
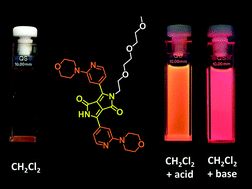
Four diketopyrrolopyrroles have been synthesized starting from heterocyclic aromatic nitriles. It was found that the negative influence of electron-donating groups on the reactivity of nitriles can be overcome by the presence of an electron-deficient pyridine ring. The absorption and emission properties of the diketopyrrolopyrroles and their N-substituted derivatives were evaluated in a range of solvents revealing that the exact position of the electron-donating substituents significantly modulated their fluorescence response. The presence of a dialkylamino moiety at position 3 of the aryl substituents led to the occurrence of very fast nonradiative deactivation processes. Formation of both the anion (located on the core) and cation (located on the pyridine ring) changes the relative energy of the excited states leading to strong red fluorescence. On the other hand, the presence of a pyrrole moiety at position 4 of the aryl substituents resulted in a record high fluorescence quantum yield (0.88). The combination of the two dialkylamino-pyridine moieties and the oligoethylene glycol substituent made it possible to obtain a compound possessing reasonable water-solubility, which was applied in fluorescence microscopy for the selective staining of mitochondria in living cells.
- This article is part of the themed collection: Shape-Responsive Fluorophores

 Please wait while we load your content...
Please wait while we load your content...