Controlling and revealing the trap distributions of Ca6BaP4O17:Eu2+,R3+ (R = Dy, Tb, Ce, Gd, Nd) by codoping different trivalent lanthanides
Abstract
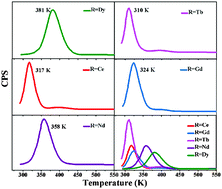
Thermoluminescence (TL) glow curves of Ca6BaP4O17:Eu2+,R3+ (R = Dy, Tb, Ce, Gd, Nd) samples were measured above room temperature in order to compare the trap distributions in the band gap. The observed phenomenon indicates that R3+ ions (R = Dy, Tb, Ce, Gd, Nd) have different effects on the trap properties of the Ca6BaP4O17:Eu2+ phosphor. The most shallow trap (0.620 eV) for the Tb3+ ion and the deepest trap (0.762 eV) for the Dy3+ ion eventually led to shorter duration (4.3 h and 1.2 h, respectively), while the appropriate trap depth (0.716 eV) for the Nd3+ ion makes the Ca6BaP4O17:Eu2+,Nd3+ sample show the longest afterglow duration (37.9 h). Codoping the Tb3+ ion slightly increases the instinct traps of the Ca6BaP4O17:Eu2+ sample and creates a new low-temperature TL peak corresponding to a relatively shallow trap leading to the strongest initial afterglow brightness (0.887 cd m−2), while codoping with other R3+ ions (R = Dy, Ce, Gd, Nd) creates new appropriate or inappropriate traps. By recording a series of long-lasting phosphorescence (LLP) spectra with various irradiated times and TL experiments with varying delay time after ceasing the UV irradiation, the trap distribution of the depth and shape was evaluated. The result provides a better understanding of the role of these trapping centers played in the persistent luminescence mechanism.

 Please wait while we load your content...
Please wait while we load your content...