Thermal stability of Mg2Si0.3Sn0.7 under different heat treatment conditions†
Abstract
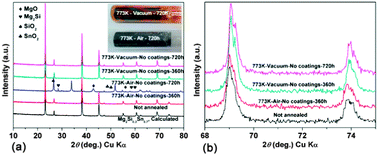
Sb-doped Mg2Si0.3Sn0.7 solid solutions were prepared by a two-step solid state reaction method followed by electron-discharge plasma activated sintering (Ed-PAS). Thermal stability was tested by changing heat treatment conditions, i.e., annealing temperature, annealing time, annealing atmosphere and preventive coatings. Mg loss is severe when the solid solutions are annealed in vacuum, due to the high saturated vapor pressure of Mg. As a consequence of Mg loss, the β Sn–Sb alloy formed. However, the solid solutions are oxidized when annealed in air. And this is effectively prevented when the samples are coated with boron nitride (BN) spray. The results showed that Mg2Si1−xSnx can be exposed for long periods of time to temperature up to about 823 K, provided it is protected with specific coatings. However, the structure becomes unstable when the temperature exceeds much beyond 823 K, mainly due to the peritectic reaction. The composition, microstructure and thermoelectric (TE) properties of the annealed samples were carefully explored and critically assessed.
- This article is part of the themed collection: The Chemistry of Thermoelectric Materials

 Please wait while we load your content...
Please wait while we load your content...