The fast and reversible intrinsic photochromic response of hydrated tungsten oxide nanosheets†
Abstract
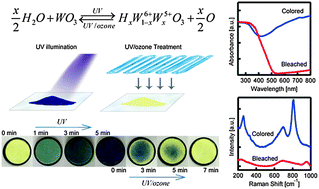
Many previous reports have indicated that the applicable photochromism of WO3 can only be achieved by combining with metals, semiconductors or organic polymers to improve the coloration efficiency and reversibility, while the investigation of intrinsic WO3 photochromism has been severely prohibited by its small color change and slow coloration rate. Herein, we report hydrothermally prepared hydrated tungsten oxide nanosheets with fast and reversible intrinsic photochromic response. A gradual yellow-blue color change can be modulated with continuous UV illumination. The following UV/ozone treatment can bleach the nanosheets back to their initial yellowish color within minutes and the reversible process can be continued 20 times without noticeable attenuation, which has never been reported for intrinsic WO3 photo response. The thin-layered nanostructure of WO3 indicates a short ionic diffusion length for surface attached species, which promotes fast surface water decomposition upon UV irradiation and accelerates proton intercalation/deintercalation, thus speeding up both the coloration and the bleaching processes. Our research elucidates that hydrated nanostructured WO3 can present significantly improved photochromic response even without WO3/metal, WO3/semiconductor or WO3/polymer combination, which may lead to technological advances with simple, facilely prepared WO3 nanosheets in areas such as photolithography, rewritable optical memory devices and nanoinks in inkjet printing.

 Please wait while we load your content...
Please wait while we load your content...