Strong solid emission and mechanofluorochromism of carbazole-based terephthalate derivatives adjusted by alkyl chains†
Abstract
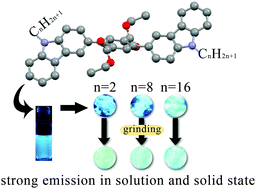
Three 2,5-dialkylcarbazole-substituted terephthalate derivatives, in which carbazole and ethoxylcarbonyl groups are used as electron-donating and -accepting moieties, respectively, were synthesized. Owing to the presence of steric hindrance between ethoxylcarbonyl and carbazole groups, three compounds show intense blue fluorescence in both solution and the solid state. The fluorescence quantum yields of compounds with octyl and hexadecyl groups in the solid state exceed 95%. Single-crystal structures of three compounds were obtained and used to interpret the strong emission in the solid state. More interestingly, three compounds exhibited alkyl length-dependent mechanofluorochromism. The compound with ethyl groups exhibited the largest spectral shift under force stimuli, but that with a hexadecyl moiety did not change its emission color after grinding. Because of strong fluorescence in solution and the solid state, we believe that they can be used as luminescent materials and sensors.

 Please wait while we load your content...
Please wait while we load your content...