Atomic layer deposition of B-doped ZnO using triisopropyl borate as the boron precursor and comparison with Al-doped ZnO†
Abstract
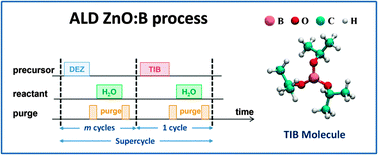
Doped ZnO films are an important class of transparent conductive oxides, with many applications demanding increased growth control and low deposition temperatures. Therefore, the preparation of B-doped ZnO films by atomic layer deposition (ALD) at 150 °C was studied. The B source was triisopropyl borate, B(OiPr)3 (TIB), which has a significantly lower vapour pressure and is a safer alternative precursor to highly toxic diborane(6), B2H6. The doping fraction (DF) of the films was varied by the ratio of ZnO and dopant ALD cycles. The electrical, structural and optical properties of the ZnO:B films were studied as a function of the dopant concentration and deposition temperature, and were compared with ZnO:Al films, where dimethylaluminium isopropoxide, [Al(CH3)2(OiPr)]2 (DMAI) and trimethylaluminium, Al2(CH3)6 (TMA) were the Al sources. A low resistivity of 3.5 mΩ cm was achieved for 45 nm-thick ZnO:B deposited at 150 °C with a doping fraction (DF) of 0.016, which was similar to the results obtained for ZnO:Al films prepared with DMAI and lower compared to the 8 mΩ cm achieved for ZnO:Al prepared with TMA at an optimized DF of 0.040. Hence TIB, as well as DMAI, outperformed the conventionally employed TMA in terms of doping efficiency at 150 °C. It was found that the optical band gap could be easily tuned over the range of ∼3.2–3.7 eV by modifying the doping fraction.


 Please wait while we load your content...
Please wait while we load your content...