Independent chemical/physical role of combustive exothermic heat in solution-processed metal oxide semiconductors for thin-film transistors†
Abstract
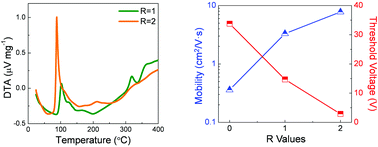
The development of high performance, solution-processed metal-oxide semiconductors has been of paramount interest in various fields of electronic applications. Among the variety of methodologies for synthesizing solution-processed precursor solutions, the combustion chemistry reaction, which involves an internal exothermic heat reaction, has drawn a tremendous amount of attraction as one of the most viable chemical approaches. In this paper, we report the synthesis of new zinc–tin oxide (ZTO) precursor solutions that can be used to independently adjust the amount of combustive exothermic heat. Through comparative analyses based on X-ray photoelectron spectroscopy, spectroscopic ellipsometry, and X-ray absorption spectroscopy, the independent influence of combustive heat is elucidated in indium-free, solution-processed oxide semiconductors, in conjunction with an interpretation of observed variations in the device performance.

 Please wait while we load your content...
Please wait while we load your content...