A highly selective ratiometric detection of F− based on excited-state intramolecular proton-transfer (imidazole) materials†
Abstract
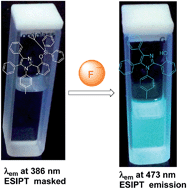
Inspired by the importance of fluoride ions in the field of dental fluorosis and treatment of osteoporosis, we have synthesized a probe 2 (2-(2-tert-butyldiphenylsiloxy)phenyl-4,5-diphenyl-1-p-tolyl-1H-imidazole) for the detection of fluoride ions from simple starting materials. The probe was characterized using standard analytical and spectroscopic techniques including single crystal X-ray crystallography. The probe (2) exhibited a multi-channel rapid response and excellent selectivity and sensitivity towards fluoride ions through selective cleavage of Si–O bonds. Time-dependant density functional theory (TD-DFT) calculations were carried out to vindicate the optical properties of the probe (2) and its starting material (compound 1).

 Please wait while we load your content...
Please wait while we load your content...