Alkyl-triphenylamine end-capped triazines with AIE and large two-photon absorption cross-sections for bioimaging†
Abstract
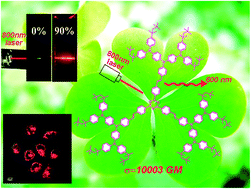
In the present study, three new luminogens ATT-(1–3) based on 1,3,5-triazine and end-capped with multi-branched triphenylamine-containing alkyl chains have been synthesized and characterized. All the three dyes are nonemissive in solution but have a strong red fluorescent emission in the aggregate state. The two-photon absorption (2PA) cross sections measured by the open aperture Z-scan technique are determined to be as large as 2756, 4750 and 10 003 GM for ATT-1, ATT-2 and ATT-3 in chloroform, respectively, showing a dramatic enhancement with an increasing number of donor branches. The relationship between their structures and properties on one- and two-photon absorption and aggregation-induced emission (AIE) is discussed, which can serve as a guideline for the development of a series of solid materials with larger two-photon cross sections and high fluorescence quantum yield. In addition, one- and two-photon fluorescence (2PF) microscopy images of HeLa cells incubated with these three dyes were obtained to demonstrate the potential applications of these fluorophores in biosensing and bioimaging.

 Please wait while we load your content...
Please wait while we load your content...