Boron-dipyrromethene based multi-anionic sensor and a specific cationic sensor for Fe3+†
Abstract
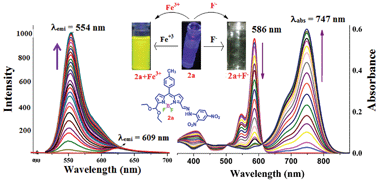
Symmetrical and unsymmetrical phenylhydrazone substituted boron-dipyrromethenes were synthesized by treating 3,5-diformyl boron-dipyrromethene with phenylhydrazine/2,4-dinitrophenylhydrazine in ethanol at reflux temperature. The X-ray structure of the unsymmetrical boron-dipyrromethene (BODIPY) was shown to have an almost extended planar orientation of BODIPY with 2,4-dinitrophenylhydrozone unit at the 3-position and ethyl acetal at the 5-position with small torsional angles. A strong absorption band at ∼600 to 700 nm and a weak emission band at ∼620 to 710 nm were observed for the phenylhydrazone substituted BODIPYs. The compounds showed interesting absorption properties in DMSO and DMF solvents compared to other solvents by shifting ∼150 nm towards the red region. Anion binding studies indicated that the unsymmetrical phenylhydrazone substituted BODIPY could be applied as a sensor for F−, CH3COO− and H2PO4− ions as confirmed by various spectroscopic studies. Furthermore, the unsymmetrical phenylhydrazone substituted BODIPY had the unique ability of acting as a two step fluorescence enhanced chemodosimetric sensor for Fe3+ ion.

 Please wait while we load your content...
Please wait while we load your content...