Dithienonaphthothiadiazole semiconductors: synthesis, properties, and application to ambipolar field effect transistors†
Abstract
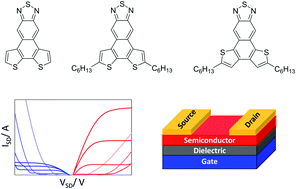
To address the scarcity of small molecule semiconductors capable of ambipolar charge injection/transport in field effect transistors, three new dithienonaphthothiadiazoles 10, 11 & 12 with fused donor/acceptor motif were synthesized. Their optical and electrochemical properties were investigated in parallel to DFT calculations, revealing the effects of ring connectivity and alkyl substitution on the electronics of the push–pull systems. Single-crystal X-ray analysis revealed a transition from a quasi-herringbone structure in 10 to slipped π-stacked structures in 11 and 12, which changes the exciton delocalization and charge transport pathways. Accordingly, while alkylated compounds 11 and 12 were insulating, ambipolar charge transport was achieved in field-effect transistor devices using 10 with balanced hole and electron mobilities of up to ∼0.02 cm2 V−1 s−1 in single-crystal devices.

 Please wait while we load your content...
Please wait while we load your content...