Novel blue-emitting Eu2+-activated LaOCl:Eu materials
Abstract
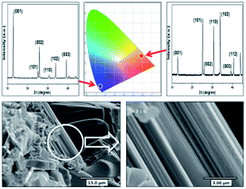
A novel blue-emitting LaOCl:Eu2+ phosphor has been successfully synthesized using the solid-state reaction. In addition, Ce3+ and Tb3+ activated LaOCl phosphors have been prepared and compared with the LaOCl:Eu2+ phosphor. FE-SEM images clearly indicate that the LaOCl:Eu2+ phosphor has a lamellar structure. XRD results and PL measurements for the LaOCl:Eu2+ phosphor indicate a preferred orientation along the [001] direction, which results in the blue-emission associated with the spectral characteristics of Eu2+. In contrast, the red-emission due to the formation of Eu3+ appears in a non-oriented one. These results reveal that Eu2+ ions are exclusively stabilized into the (00l) oriented LaOCl crystal lattice because the excitation and emission spectra of the blue-emitting LaOCl:Eu2+ phosphor consist of the spectral characteristics of Eu2+. The CIE chromaticity index (x = 0.151, y = 0.048) with high color saturation indicates that it is a promising candidate as a blue-emitting phosphor for white-light UV-LEDs.

 Please wait while we load your content...
Please wait while we load your content...