Growth evolution and characterization of ultra-thin CoGe2 films synthesized via a catalytic solid–vapour reaction technique†
Abstract
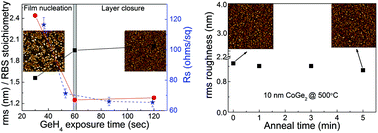
A low temperature process, relying on the catalytic chemical vapor reaction of GeH4 with solid Co layers, is developed to synthesize ultra-thin CoGe2 layers (10–20 nm) on 300 mm Si wafers. The selective reaction of Co with GeH4 results in the direct formation of CoGe2 films crystallizing in the orthorhombic structure. Detailed studies under germanidation reaction conditions show that the optimal temperature (325 °C vs. 400 °C) to obtain low resistive, defect free and continuous CoGe2 films depends on the initial Co thickness. Investigation on various stages of cobalt germanide growth evolution demonstrates that the complete conversion of Co, leading to closed and stoichiometric CoGe2 films, occurs after 60 s of GeH4 exposure as evidenced by atomic force microscopy (AFM), Rutherford back scattering (RBS) and sheet resistance (Rs) analysis. The CoGe2 formation is found to exhibit self-limiting growth. The layers demonstrate constant film properties (phase purity, resistivity, composition homogeneity, uniformity, and smooth morphology with insignificant rms roughness increase) within a large process window, negligibly influenced by excessive GeH4 exposure conditions. Independent of the post-deposition annealing employed (RTA or furnace annealing), results showed the CoGe2 layers to be thermally stable up to 500 °C without phase or morphological degradation or film roughening. Resistivity values in the range of 70–50 μΩ cm were observed for 10–20 nm CoGe2 films.

 Please wait while we load your content...
Please wait while we load your content...