Electroactive poly(oligoaniline)/gold nanocomposite thin films prepared by simultaneous solid-state deprotection and redox reaction†
Abstract
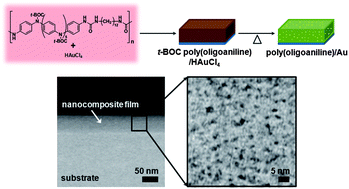
We prepared electroactive nanocomposite films consisting of poly(oligoaniline)s and Au nanoparticles via simultaneous deprotection and redox chemistry performed in the solid state. Thermal treatment of a mixture composed of tert-butoxycarbonyl-protected poly(oligoaniline) and chloroauric acid led to the reduction of Au(III) to Au(0) and the simultaneous deprotection and oxidation of the polymer. The reactivity between poly(oligoaniline) and chloroauric acid was controlled by varying several reaction conditions, yielding Au nanoparticles with a diameter of several nanometers dispersed uniformly in the poly(oligoaniline) matrix. The presence of electrostatic interaction between the polymers and the Au nanoparticles gave enhanced electrical conductivity and electrochemical activity as compared with pure polymers. The use of a precursor polymer containing multiple oligoaniline blocks and in situ generation of metal nanoparticles demonstrated here are promising for the creation of new electroactive functional polymeric materials.

 Please wait while we load your content...
Please wait while we load your content...