Going from green to red electroluminescence through ancillary ligand substitution in ruthenium(ii) tetrazole benzoic acid emitters†
Abstract
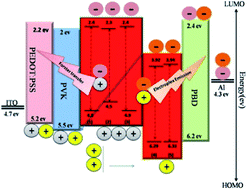
The synthesis, characterization, photoluminescence (PL) and electroluminescence (EL) properties of novel ruthenium(II) emitters with 4-(1H-tetrazole-5-yl)benzoic acid (TzBA) as a novel basic ligand and 2,2-bipyridine (bpy), 1,10-phenanthroline (phen) and pyridine tetrazole (pyTz) as ancillary ligands have been reported. The EL results show that the luminescence of Ru(TzBA) depends on the ancillary ligands, which can be used to tune the EL color of the Ru(TzBA) complexes from green to red emission. The role of ancillary ligands in EL devices of the Ru(TzBA) complexes was investigated by DFT calculations. The device [Ru(TzBA)(bpy)(pyTz)(SCN)] (5) has a luminance of 480 cd m−2 and a maximum efficiency of 1.2 cd A−1 at 16 V, which are the highest values among the five devices studied. The turn-on voltage of this device is approximately 6 V. We suggest that an electroplex occured at the PVK–Ru complex interface when pyTz was incorporated as an ancillary ligand into Ru(TzBA). OLED studies reveal that pyTz as an ancillary ligand exhibits better current generating capacity than bpy and phen ligands, which are introduced by an emitter (5).

 Please wait while we load your content...
Please wait while we load your content...