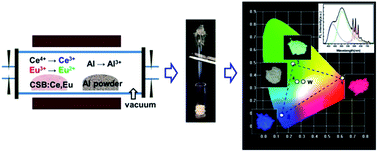
Ce3+, Eu2+ and Eu3+ singly activated and Ce3+/Eu2+/Eu3+-coactivated Ca11(SiO4)4(BO3)2 (CSB) phosphors were synthesized by novel and efficient aluminium reduction solid state reactions. Under ultraviolet light excitation, the Ce3+, Eu2+ and Eu3+ singly activated CSB phosphors exhibit intense red, green and blue emissions, respectively. The unique host CSB shows its superior advantages in color balance compared with the widely-used Ca2SiO4, Ca3B2O6 and Y3Al5O12. The red, green and blue colors can be controlled by the reduction temperature, and a white light emitting phosphor was obtained by reducing the as-prepared CSB:0.02Ce,0.005Eu powder at 700 °C for 8 h. The unique property of CSB in color balance was proposed to attribute to the group synergy effect. Furthermore, the energy transfer probability was discussed in detail, and the critical distance was calculated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...