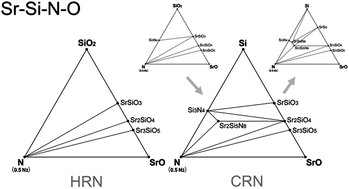
Thermodynamic stabilities of the phases of Sr–Si–N–O system were evaluated by simulating phase diagrams at various conditions based on first-principles density functional theory calculations. Synthesis conditions and stability of the compounds belonging to the system, in which oxidation and nitridation reactions are involved complicatedly, could be interpreted through this first systematic investigation on the two-gas system. Practical synthetic methods of nitrides, such as hydrogen-reduction and nitridation or carbothermal reduction and nitridation reactions, were studied with special attention. This study enabled us to calculate proper conditions for synthesis of the Sr2Si5N8 phase, which is drawing attention as a new phosphor material for light emitting diodes. The types of impurities appearing with deviation from the proper synthetic conditions were also investigated, which may provide information about optimizing synthesis conditions. Synthesis of Sr2Si5N8:Eu phosphor using SiO2, instead of conventionally used Si3N4, was predicted by first-principles calculations, and we succeeded in synthesizing Sr2Si5N8:Eu phosphor for the first time using all oxide raw materials under normal pressure on this basis. The results of this study are expected to provide useful guidelines for synthesis of nitrides and the established simulation method may effectively be applied to other multi-gas systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...