Enzyme mimics based on self-assembled peptides for di(2-ethylhexyl)phthalate degradation†
Abstract
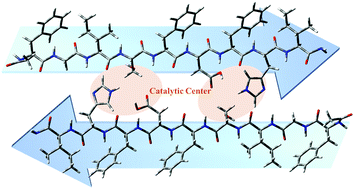
Enzyme mimics have been developed by imitating and incorporating specific features of native enzymes to achieve catalytic activity, and are expectedly comparable to that of native enzymes. Here, inspired by the “catalytic triad” in serine proteases, a series of peptide-based enzyme mimics were designed to follow the rational design principle of peptides via self-assembly, and were further applied in the degradation of di(2-ethylhexyl)phthalate (DEHP). The relationship of the structure of enzyme mimics with their degradation activity was analyzed by transmission electron microscopy, fluorescence spectroscopy, circular dichroism, Raman spectroscopy, X-ray diffraction spectroscopy, and computational modeling. These results show that the hydrophobic skeleton, amino acid sequence, species, and periodic distribution have important effects on the structure of the peptide sequence and the number of hydrogen bonds; in addition, pH can also affect the self-assembly characteristics of peptides and the formation of stable fibers, which are all closely linked to the catalytic activity of the enzyme mimics. The self-assembled peptides had a stable fibrous morphology and secondary structure after the DEHP degradation assay. The enzyme mimics with high catalytic activity constructed from the self-assembled peptides may provide guidance for the future degradation of DEHP in food packaging or water treatment, and also give insights into the design of enzyme mimics in other related fields.


 Please wait while we load your content...
Please wait while we load your content...