Programmable enzymatic oxidation of tyrosine–lysine tetrapeptides†
Abstract
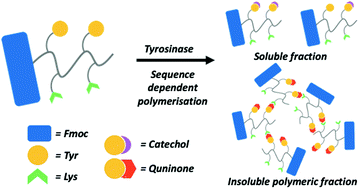
The ability to control the response of self-assembled systems upon exposure to external stimuli has been a long-standing goal of supramolecular chemistry. Short peptides are an attractive platform to realise this objective due to their chemical diversity and modular nature. Here, we synthesise a library of Fmoc-capped tetrapeptides, each containing two tyrosine and two lysine residues and varying in their amino acid sequence. Despite having similar secondary structure, these tetrapeptides form structures which are highly sequence dependent, yielding aggregates, nanofibres or monomers. This in turn highly affects the rate and degree of oxidative polymerisation by the enzyme tyrosinase, with self-assembled nanofibres exhibiting a greater degree of polymerisation. We monitor the formation of tyrosine oxidation products over time, finding that the precipitation of polymers is driven by quinone-based species. This affects the electrochemical properties of the oxidised peptide polymers, as determined through electrical impedance spectroscopy. Finally, intrinsic fluorescence microscale thermophoresis studies confirm that the degree of oxidative polymerisation is highly dependent on tyrosine solvent accessibility and the presence of peptide monomers. The ability to tune the kinetics of enzymatically active substrates and understand their polymerisation pathways on a molecular level is important for the creation of programmable, enzyme responsive biomaterials.


 Please wait while we load your content...
Please wait while we load your content...