Surfaces immobilized with oligo-prolines prevent protein adsorption and cell adhesion†
Abstract
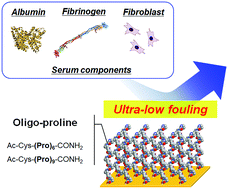
In this study, oligo-prolines, (Pro)n (n = 6 and 9) inspired by the backbone structure of collagen, were evaluated as a novel non-ionic anti-fouling peptide. Two oligo-prolines with a cysteine residue were synthesized and immobilized on gold substrates via Au–thiol binding. The surfaces immobilized with oligo-prolines, and forming a polyproline-II conformation, indicated hydrophilic properties (water contact angle ≈ 25 degrees). The degree of adsorption of human serum albumin, human fibrinogen, and bovine serum components on these surfaces was quantified using a quartz crystal. The immobilization of oligo-prolines prevented the adsorption of proteins and serum components including small molecules, such as fatty acids. Pro9 specifically indicated good resistance to the adsorption of all components due to the highly-packed Pro9 chains on the surface. The adhesion of fibroblasts was drastically suppressed on the surfaces immobilized with oligo-prolines. Our findings suggest that oligo-proline-immobilized surfaces, specifically Pro9-s, are useful for the development of novel vascular devices that have ultra-low fouling properties.


 Please wait while we load your content...
Please wait while we load your content...