Auto-catalytic redox polymerisation using nanoceria and glucose oxidase for double network hydrogels
Abstract
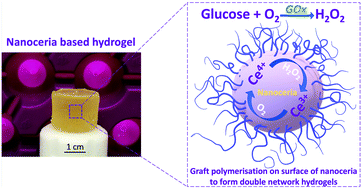
A novel auto-catalytic reaction, a combination of naturally occurring enzyme glucose oxidase (GOx) and amine-functionalised cerium oxide nanoparticles (nanoceria), was employed for open vessel free radical polymerisation of double network hydrogels (DNHGs). The nanoceria also incorporated into the gels to enhance mechanical strength. GOx reduces atmospheric O2 to H2O2, causing a cyclic change of cerium ion states, resulting in propagating free radicals in the carbon group in the amino functionalised nanoceria surface. We synthesised novel nanocomposite DNHGs by grafting polymers onto amine-functionalised nanoceria (ANC), with poly(2-acrylamido-2-methylpropanesulfonic acid), PAMPS, and polyacrylamide (PAAm) in the first and second networks respectively. The graft polymerisation was initiated using the alternating cerium states on the ANC. GOx held two major roles within the reaction: to provide an oxygen free system, without any other form of degassing, and to provide cyclical cerium ion states between Ce4+ and Ce3+, creating new free radicals for polymerisation. Polymer conversion using ANC as the sole initiator in the presence of GOx resulted in 83% conversion for PAMPS and 64% PAAm. Polymers degassed only with argon resulted in less than 55% conversion for both PAAm and PAMPS, proving that the addition of GOx enhanced the reaction. The new gels (1.76 MPa) showed an order of magnitude improvement in mechanical properties compared to DNHG made without ANC/GOx (0.10 MPa).


 Please wait while we load your content...
Please wait while we load your content...