Active-targeting and acid-sensitive pluronic prodrug micelles for efficiently overcoming MDR in breast cancer†
Abstract
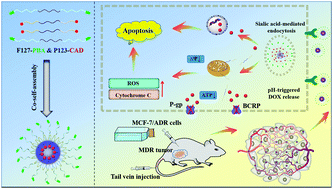
Multidrug resistance (MDR) seriously hinders therapeutic efficacy in clinical cancer treatment. Herein, we reported new polymeric prodrug micelles with tumor-targeting and acid-sensitivity properties based on two different pluronic copolymers (F127 and P123) for enhancing tumor MDR reversal and chemotherapy efficiency in breast cancer. Hybrid micelles were composed of phenylboric acid (PBA)-modified F127 (active-targeting group) and doxorubicin (DOX)-grafted P123 (prodrug groups), which were named as FBP-CAD. FBP-CAD exhibited good stability in a neutral environment and accelerated drug release under mildly acidic conditions by the cleavage of β-carboxylic amides bonds. In vitro studies demonstrated that FBP-CAD significantly increased cellular uptake and drug concentration in MCF-7/ADR cells through the homing ability of PBA and the anti-MDR effect of P123. In vivo testing further indicated that hybrid micelles facilitated drug accumulation at tumor sites as well as reduced side effects to normal organs. The synergistic effect of active-targeting and MDR-reversal leads to the highest tumor growth inhibition (TGI 78.2%). Thus, these multifunctional micelles provide a feasible approach in nanomedicine for resistant-cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...