Double network hydrogels based on semi-rigid polyelectrolyte physical networks†
Abstract
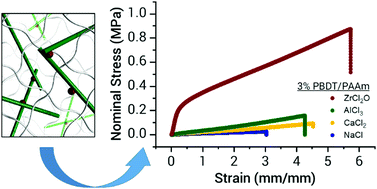
Applying the double network principle to develop tough hydrogels with different polymer chemistries is important for the potential application of hydrogel materials. Synthesis of the two interpenetrated networks with contrasting structure and properties required for double networks usually involves a two-step polymerization process. In this work, we present a new method to synthesize tough double network hydrogels by post-physical crosslinking of linear semi-rigid polyelectrolytes entrapped in a chemically crosslinked neutral network. Owing to their semi-rigid structure, the linear polyelectrolytes form a brittle physical network above their overlap concentration in multi-valent ZrCl2O ion solutions without macroscopic phase separation within the flexible neutral network. The double network hydrogels thus prepared exhibit high modulus (∼1.7 MPa), strength (∼1.3 MPa), fracture strain (∼7.3), and strain energy density (∼5.9 MJ m−3), while containing over 80% water. These materials also exhibit modest self-healing ability (∼51% after 30 minutes), demonstrating an additional benefit of a physical sacrificial network. This method is simpler than the conventional two-step polymerization and could be applied to develop tough hydrogels from rigid polyelectrolytes, including biopolymers such as DNA, HA, and chondroitin sulfate.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...