A biodegradable polyphosphoester-functionalized poly(disulfide) nanocarrier for reduction-triggered intracellular drug delivery
Abstract
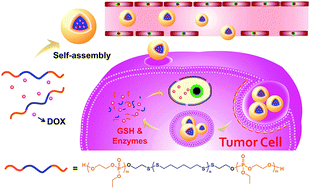
Stimuli-responsive and biodegradable polymeric carriers are of great importance for safe delivery and efficient release of chemotherapeutic agents. In this work, given the unique advantages of poly(disulfide)s and biodegradable polyphosphoesters, we designed and constructed a reduction-sensitive amphiphilic triblock copolymer poly(ethyl ethylene phosphate)-b-poly(disulfide)-b-poly(ethyl ethylene phosphate) (PEEP-PDS-PEEP) by combining thiol-disulfide polycondensation and ring-opening polymerization (ROP). The thiol–disulfide polycondensation between 1,6-hexanedithiol and 2,2′-dithiodipyridine yielded the linear telechelic pyridyl disulfide-terminated poly(disulfide)s, followed by the treatment with 2-mercaptoethanol to quantitatively produce dihydroxyl-terminated poly(disulfide)s, which was used to initiate the ROP reaction of 2-ethoxy-2-oxo-1,3,2-dioxaphospholane, generating ABA-type amphiphilic triblock copolymers. The chemical structures of various polymers were thoroughly characterized and verified using nuclear magnetic resonance (NMR) spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, gel permeation chromatography (GPC) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy. The resultant amphiphilic PEEP-PDS-PEEP could self-assemble into spherical nanoparticles in aqueous solution as evidenced from dynamic light scattering (DLS) and transmission electron microscopy (TEM) analyses. Hydrophobic anti-tumor drug doxorubicin (DOX) was used to study the encapsulation capacity of nanoparticles, the drug loading content (DLC) and drug loading efficiency (DLE) values were determined to be 11.2% and 31.5%, respectively. In vitro release studies indicated that DOX was released much faster under reductive conditions compared to physiological conditions, confirming their reduction-responsive release behavior owing to the scission of the poly(disulfide) segment and subsequent disintegration of nanoparticles. The cellular uptake study using a live cell imaging system demonstrated that this DOX-loaded nanoparticle can be internalized into HeLa cells and release DOX over time. Methyl thiazolyl tetrazolium (MTT) assay revealed the favorable cytocompatibility of a bare triblock copolymer toward both L929 and HeLa cells, whereas the DOX-loaded copolymer nanoparticles exhibited the lower inhibitory ability against HeLa and HepG2 cell proliferation than free DOX. This finding presents a strategy for the construction of biocompatible and reduction-responsive polymeric drug carriers.
- This article is part of the themed collection: Journal of Materials Chemistry B Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...