Orthogonal click reactions enable the synthesis of ECM-mimetic PEG hydrogels without multi-arm precursors†
Abstract
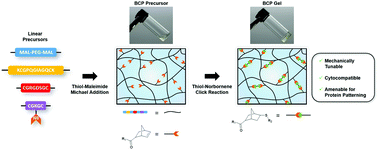
Click chemistry reactions have become an important tool for synthesizing user-defined hydrogels consisting of poly(ethylene glycol) (PEG) and bioactive peptides for tissue engineering. However, because click crosslinking proceeds via a step-growth mechanism, multi-arm telechelic precursors are required, which has some disadvantages. Here, we report for the first time that this requirement can be circumvented to create PEG–peptide hydrogels solely from linear precursors through the use of two orthogonal click reactions, the thiol–maleimide Michael addition and thiol–norbornene click reaction. The rapid kinetics of both click reactions allowed for quick formation of norbornene-functionalized PEG–peptide block copolymers via Michael addition, which were subsequently photocrosslinked into hydrogels with a dithiol linker. Characterization and in vitro testing demonstrated that the hydrogels have highly tunable physicochemical properties and excellent cytocompatibility. In addition, stoichiometric control over the crosslinking reaction can be leveraged to leave unreacted norbornene groups in the hydrogel for subsequent hydrogel functionalization via bioorthogonal inverse-electron demand Diels–Alder click reactions with s-tetrazines. After selectively capping norbornene groups in a user-defined region with cysteine, this feature was leveraged for protein patterning. Collectively, these results demonstrate that our novel chemical strategy is a simple and versatile approach to the development of hydrogels for tissue engineering that could be useful for a variety of applications.


 Please wait while we load your content...
Please wait while we load your content...
