A low background D–A–D type fluorescent probe for imaging of biothiols in living cells†
Abstract
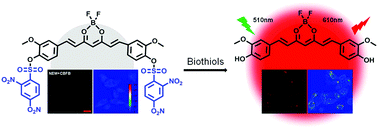
Two probes, structurally symmetric CBFB and asymmetric CBFM, constructed by a D–A–D (donor–acceptor–donor) type curcuminoid as the fluorophore and the DNBS (2,4-dinitrobenzenesulfonyl) group as the biothiol recognition site were designed and synthesized here. The DNBS group can quench the emission of the fluorophore by the PET (photoinduced electron transfer) process, and in the presence of biothiols, the emission of the probe was switched on as a result of the cleavage of the quencher by a nucleophilic aromatic substitution reaction. Experimental analyses and theoretical calculations revealed that two recognition moieties in the molecule can quench the fluorescence more efficiently, therefore, CBFB showed a much higher SNR (signal to noise ratio) than CBFM in biothiol detection with an emission maximum at 610 nm. This “low background” and “turn-on” fluorescent probe, CBFB, was successfully utilized to map endogenous biothiols in living cells.


 Please wait while we load your content...
Please wait while we load your content...