Photoluminescent and pH-responsive supramolecular structures from co-assembly of carbon quantum dots and zwitterionic surfactant micelles†
Abstract
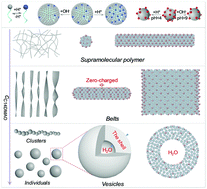
The co-assembly of negatively charged carbon quantum dots (CQDs) and a zwitterionic surfactant (tetradecyldimethylamine oxide, C14DMAO) in water has been investigated, which results in the formation of a new class of photoluminescent and pH-responsive self-assemblies. Instead of a direct binding mode, the surfactant unimers self-associate into micelles before interacting with the CQDs. Each of the micelles carries a small amount of positive charge due to the partial protonation of C14DMAO (C14DMAO + H+ = C14DMAOH+). For aqueous solutions containing 0.1 mg mL−1 CQDs, two types of structures have been confirmed upon the addition of C14DMAO, i.e., supramolecular polymers and vesicles. Between them, a variety of intermediate structures are observed, including ribbons, oligomers of vesicles and helixes. Due to the reversible protonation/deprotonation of the zwitterionic surfactant, both the supramolecular polymers and vesicles exhibit reversible changes toward the variation of pH, providing opportunities to facilely adjust their properties wherever necessary. As the CQDs evenly distribute in the micelle matrix, the self-quenching of the CQDs has been significantly suppressed and their photoluminescence has been well preserved. The supramolecular polymers have been further selected as hosts to accommodate rhodamine 6G, which is used as a model drug, and the controlled release stimulated by pH has been realized.


 Please wait while we load your content...
Please wait while we load your content...