Fabrication and biomedical applications of AIE active nanotheranostics through the combination of a ring-opening reaction and formation of dynamic hydrazones†
Abstract
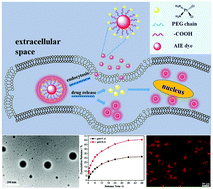
Aggregation-induced emission (AIE) dyes based on fluorescent organic nanoparticles (FONs) have attracted increasing interest over the past few years. However, the biomedical applications of AIE dyes based on FONs for simultaneous biological imaging and therapeutic applications have rarely been reported thus far. In this study, an amino group terminated phenothiazine (named as ATPHE) with AIE features and red fluorescence was synthesized and utilized for the fabrication of AIE active FONs via a facile one-pot strategy, which relied on the ring-opening reaction between ATPHE and an anhydride containing compound. Then, the keto group of the AIE active polymeric intermediate was subsequently conjugated with hydrazide terminated polyethylene glycol (HTPEG) through the formation of hydrazone bonds. These amphiphilic AIE active copolymers are readily self-assembled into nanoscale particles in an aqueous solution, which resulted in strong luminescence and good water dispersibility of the final HTPEG@ATPHE-co-BTDA FONs. The excellent physicochemical and biological properties of HTPEG@ATPHE-co-BTDA FONs give them high potential for biological imaging and controlled drug delivery applications. Taken together, we developed a simple strategy for the fabrication of AIE active nanoparticles, which are promising for biological imaging and controlled drug delivery.

 Please wait while we load your content...
Please wait while we load your content...