Selective sensing of isoprene by Ti-doped ZnO for breath diagnostics
Abstract
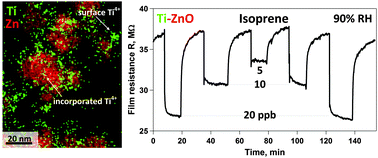
Exhaled isoprene could enable non-invasive monitoring of cholesterol-lowering therapies. Here, we report an isoprene-selective sensor at high relative humidity (RH) for the first time (to our knowledge). It is made of nanostructured, chemo-resistive Ti-doped ZnO particles (10–20 nm crystal size) produced by flame spray pyrolysis (FSP) and directly deposited in one step onto compact sensor substrates forming highly porous films. The constituent particles consist of stable Ti-doped ZnO solid solutions for Ti levels up to 10 mol% apparently by substitutional incorporation of Ti4+ into the ZnO wurtzite lattice and dominant presence at the particle surface. These Ti4+ point defects strongly enhance the isoprene sensitivity (>15 times higher than pure ZnO) and turn ZnO isoprene-selective, while also improving its thermal stability. In situ infrared spectroscopy confirms that Ti4+ intensifies the surface interaction of Ti-doped ZnO with isoprene by providing additional sites for chemisorbed hydroxyl species. In fact, at an optimal Ti content of 2.5 mol%, this sensor shows superior isoprene responses compared to acetone, NH3 and ethanol at 90% RH. Most notably, breath-relevant isoprene concentrations can be detected accurately down to 5 ppb with high (>10) signal-to-noise ratio. As a result, an inexpensive isoprene detector has been developed that could be easily incorporated into a portable breath analyzer for non-invasive monitoring of metabolic disorders (e.g. cholesterol).

 Please wait while we load your content...
Please wait while we load your content...