Rationally designed two-photon absorption compounds based on benzoxazole derivatives: structure–property relationships and bio-imaging applications†
Abstract
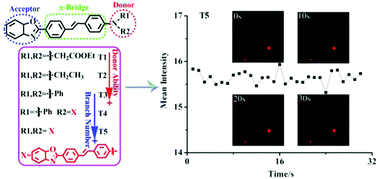
A new series of benzoxazole-based two-photon absorption (2PA) chromophores (T1–5) were synthesized and fully characterized. Among them, T1–3 are linear donor (D)–π–acceptor (A) type fluorophores with various electron-donating groups, while T4 and T5 are multi-branched compounds stemming from T3. Their photophysical properties have been systematically investigated, and the two-photon absorption properties indicate that the highest 2PA cross-section (σ2PA) is 2702 GM for T5 (in DMF). Structure–property relationships of the chromophores are comprehensively discussed based on their optical properties, crystal structures and density functional theory (DFT) calculations. In addition, a two-photon fluorescence cell imaging experiment demonstrates the potential bio-application of T1–5 with good photostability and low cytotoxicity.
- This article is part of the themed collection: JMC B Editor’s choice web collection: ‘‘seeing the unseen updated: advances in bioimaging’’

 Please wait while we load your content...
Please wait while we load your content...