A synergic nanoantioxidant based on covalently modified halloysite–trolox nanotubes with intra-lumen loaded quercetin†
Abstract
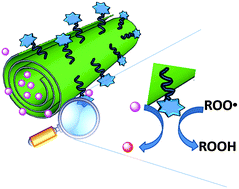
We describe the preparation and properties of the first example of a synergic nanoantioxidant, obtained by different functionalizations of the external surface and the inner lumen of halloysite nanotubes (HNTs). Trolox, a mimic of natural α-tocopherol, was selectively grafted on the HNT external surface; while quercetin, a natural polyphenolic antioxidant, was loaded into the inner lumen to afford a bi-functional nanoantioxidant, HNT–Trolox/Que, which was investigated for its reactivity with transient peroxyl radicals and a persistent 1,1-diphenyl-2-picrylhydrazyl (DPPH˙) radical in comparison with the corresponding mono-functional analogues HNT–Trolox and HNT/Que. Both HNT–Trolox and HNT/Que showed good antioxidant performance in the inhibited autoxidation of organic substrates; however HNT–Trolox/Que protection by reaction with peroxyl radicals was 35% higher in acetonitrile and 65% in chlorobenzene, as compared to the expected performance based on the sum of contributions of NHT-Trolox and NHT/Que. Similar enhancement was observed also in the trapping of DPPH˙ radicals. Synergism between the distinct antioxidant functions was based on the rapid reaction of externally exposed Trolox (rate constant with peroxyl radicals was 1.1 × 106 M−1 s−1 and 9 × 104 M−1 s−1 respectively in chlorobenzene and acetonitrile, at 30 °C), followed by its regeneration by quercetin released from the HNT lumen. The advantages of this novel nanoantioxidant are discussed.

 Please wait while we load your content...
Please wait while we load your content...