Substrate-independent, Schiff base interactions to fabricate lysine-functionalized surfaces with fibrinolytic activity†
Abstract
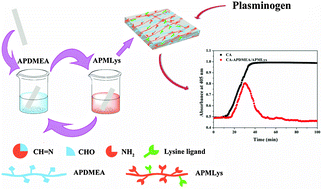
Mimicking natural fibrinolytic mechanisms that covalently bind lysine-ligands (free ε-amino and carboxylic groups) onto biomaterial surfaces is an attractive strategy to prevent clot formation on blood contact materials. However, the modification process is typically complicated and limited due to the diversity of biomaterials. Herein, we describe a simple, substrate-independent protocol to prepare a lysine-ligand functionalized layer on biomaterial surfaces. This approach is based on the adsorption and cross-linking of aldehyde-functionalized poly(N-(2,2-dimethoxyethyl)methacrylamide) (APDMEA) and amino-functionalized polymethacryloyl-L-lysine (APMLys) on a variety of substrates, such as polyurethane (PU), polydimethylsiloxane (PDMS), polyvinylchloride (PVC), stainless steel (SS) and cellulose acetate (CA). The lysine-ligand functionalized layer on substrates highly enhanced the specific adsorption of plasminogen from plasma and showed good chemical stability and excellent biocompatibility with L929 cells using the MTT assay. Moreover, for example, after the adsorbed plasminogen was activated and converted into plasmin, the fibrinolytic functionalization of CA was demonstrated using a modified plasma recalcification assay. Collectively, considering the advantages of simplicity, environmental friendliness and substrate-independence, the present study might therefore represent a general approach for the construction of a biointerface with fibrinolytic activity.

 Please wait while we load your content...
Please wait while we load your content...