Flexible cyclic siloxane core enhances the transfection efficiency of polyethylenimine-based non-viral gene vectors†
Abstract
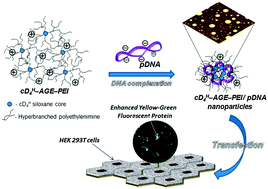
Transfection of nucleic acid molecules, large enough to interfere with the genetic mechanisms of active cells, can be performed by means of small carriers, able to collectively collaborate in generating cargocomplexes that could be involved in passive mechanisms of cellular uptake. The present work describes the synthesis, characterization, and evaluation of transfection efficacy of a conjugate molecule, which comprises a cyclic siloxane ring (2,4,6,8-tetramethylcyclotetrasiloxane, cD4H) as the core, and, on average, 3.76 molecules of 2 kDa polyethyleneimine (PEI) as cationic branches, with an average molecular mass of 7.3 kDa. As demonstrated by in silico molecular modeling and dynamic simulation, the conjugate molecule (cD4H–AGE–PEI) tends to adopt an asymmetric structure, specific for amphipathic molecules (confirmed by a log P value of −1.902 ± 0.06), that favors a rapid interaction with nucleic acids. The conjugate and the polyplexes with the pEYFP plasmid were proved to be non-cytotoxic, and capable of ensuring transfection yields better than 30%, on HEK 293T cell culture, superior to the value obtained using the SuperFect® reagent. We presume that the increased transfection efficacy originates in the ability of the conjugate to locally tightly encompass pDNA molecules by electrostatic interaction mediated by the short PEI branches, and consequently to expose the siloxane hydrophobic moiety, which decreases the interaction energy with the lipid layers.

 Please wait while we load your content...
Please wait while we load your content...