Doxorubicin conjugated phospholipid prodrugs as smart nanomedicine platforms for cancer therapy†
Abstract
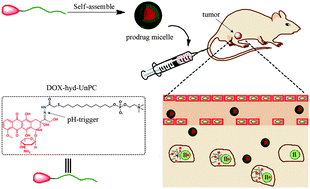
Ideal drug delivery systems should have prolonged circulation in blood, high accumulation in tumors with fast cellular uptake and burst drug release in cancer cells. Herein, a pH-sensitive small-molecule phospholipid prodrug based on phosphorylcholine (PC) was designed to overcome these challenges. The prodrug was synthesized by conjugating doxorubicin (DOX) to 11-mercaptoundecyl phosphorylcholine via an acid-labile hydrazone linker (UnPC-hyd-DOX). This phospholipid prodrug can self-assemble into core–shell micelles with exact and very high drug loading content (56.2%). Interestingly, the PC prodrug micelles can strongly minimize nonspecific phagocytosis by macrophages. They exhibited better ability to be internalized by cancer cells than that of the PEG prodrug micelles, which indicated that the PC shell facilitated cancer cell internalization. Moreover, in vitro results further demonstrated that DOX of phospholipid prodrug micelles can effectively escape from endo/lysosomes to the cytosol and enter the nucleus. An in vivo study demonstrated that these PC prodrug micelles exhibited much lower cardiotoxicity than free DOX. Importantly, PC prodrug micelles showed significantly slower blood clearance than PEG prodrug micelles and free DOX.

 Please wait while we load your content...
Please wait while we load your content...